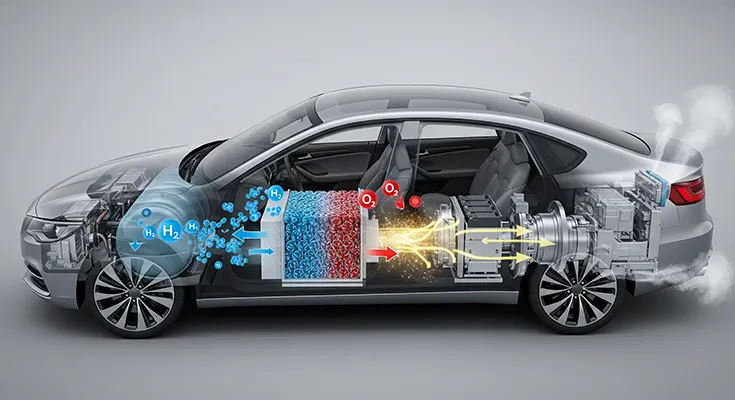
Silent Thunder: The Performance Benefits of Electric Vehicles
For decades, the sound of a roaring engine and the feel of a multi-gear transmission were the hallmarks of a high-performance car. The idea of a fast, fun-to-drive vehicle was intrinsically linked to internal combustion. However, electric vehicles (EVs) are rewriting the rules of automotive performance, offering a driving experience that is not just different, but in many ways, superior. The key to this transformation lies in two fundamental characteristics of an electric motor: instant torque and continuous acceleration. Instant Torque: The Power of a Push In a traditional gasoline car, torque—the rotational force that gets the wheels moving—is only available in a specific, narrow RPM range. When you press the accelerator, a complex series of actions must occur: fuel and air ignite, pistons fire, and a crankshaft rotates, all before power is delivered through a transmission to the wheels. This process, while seemingly fast, introduces a moment of …