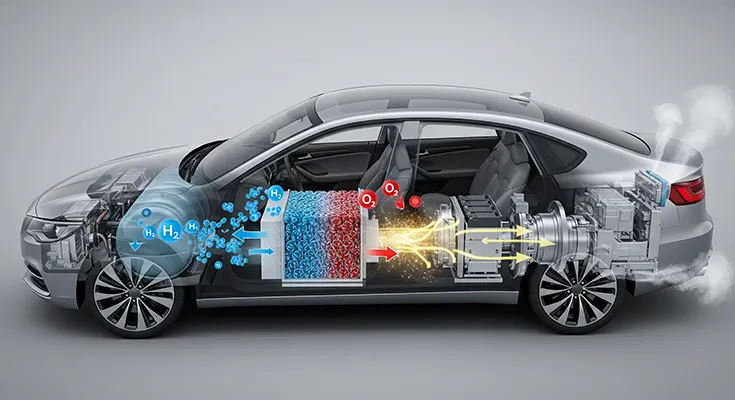
Hydrogen fuel cell vehicles (FCEVs) represent a fascinating and promising branch of the zero-emission automotive industry. Unlike battery electric vehicles (BEVs) that draw power from a large, pre-charged battery, FCEVs produce their own electricity on board, effectively acting as a small, mobile power plant. This process relies on a key chemical reaction within the heart of the car: the fuel cell stack.
How a Fuel Cell Car Works: The Simple Breakdown
At its core, a fuel cell vehicle is a type of electric car. It uses an electric motor to turn the wheels, but the source of its power is different. Here’s a step-by-step look at the process:
- Hydrogen Storage: The vehicle stores compressed hydrogen gas (H₂) in a high-pressure, reinforced tank.
- Air Intake: The car draws in oxygen (O₂) from the ambient air, typically through a front grille.
- The Fuel Cell Stack: This is where the magic happens. The fuel cell stack is made up of many individual fuel cells. Each cell contains two electrodes—an anode and a cathode—separated by a special membrane called a proton exchange membrane (PEM).
- Electrochemical Reaction:
- Hydrogen gas is channeled to the anode. A catalyst (usually platinum) at the anode’s surface splits the hydrogen molecules into protons (positively charged H+ ions) and electrons (negatively charged e-).
- The PEM allows only the protons to pass through to the cathode.
- The electrons, unable to cross the membrane, are forced to travel through an external circuit, creating an electric current. This is the electricity that powers the car’s motor.
- At the cathode, the protons, electrons, and oxygen from the air combine to form water (H2O).
- Powering the Vehicle: The electricity generated by the fuel cell stack either powers the electric motor directly or recharges a small buffer battery. This battery is used for providing extra power during acceleration and capturing energy from regenerative braking.
- The Only Emission: The only byproduct of this entire process is pure water vapor (H2O) and a little heat, which is released through the exhaust pipe. This makes FCEVs a true zero-tailpipe-emission vehicle.
A Note on the Battery
It’s important to understand that FCEVs are not entirely “battery-free.” They have a small battery that works in tandem with the fuel cell. This battery is much smaller and lighter than the one found in a BEV and serves a different purpose: it acts as a power buffer, providing a quick surge of energy for high-demand situations and storing energy recuperated during braking, which is a key part of maximizing efficiency.
Understanding Efficiency: The Energy Chain
When evaluating the efficiency of a vehicle, it’s crucial to consider the entire “well-to-wheel” energy chain—from the source of the energy to the power that moves the vehicle. This is where the debate between FCEVs and BEVs often comes into sharp focus.
- Internal Combustion Engine (ICE) Cars: A typical gasoline engine is notoriously inefficient, with only about 20-30% of the energy in the fuel being converted into power to move the car. The rest is lost as heat.
- Hydrogen Fuel Cell Vehicles (FCEVs): FCEVs are much more efficient than ICE vehicles. The fuel cell stack itself is highly efficient at converting hydrogen’s chemical energy into electricity, typically operating at 40-60% efficiency. This is a significant improvement over an ICE. However, when you look at the entire energy chain, from the production of hydrogen (especially “green” hydrogen via electrolysis) to its compression, transportation, and final use, there are multiple conversion losses.
- Battery Electric Vehicles (BEVs): BEVs have a more direct energy path. Electricity from the grid (or a renewable source) is used to charge the battery, which then powers the motor. While there are losses in charging and discharging the battery and in the grid itself, the overall “well-to-wheel” efficiency of a BEV is typically higher than that of an FCEV. Studies often cite BEV efficiency as being in the 70-80% range, while the total efficiency for an FCEV is often considered to be in the 30-40% range.
Why the Efficiency Gap Matters
This efficiency gap means that to travel the same distance, an FCEV requires more primary energy (electricity) to be produced at the source than a BEV. This is a critical factor for the widespread adoption of FCEVs, as it impacts the cost of fueling and the overall energy required for a national fleet.
However, FCEVs have their own unique advantages that may outweigh this efficiency difference in certain applications, such as their fast refueling time (3-5 minutes) and lighter vehicle weight for long-haul distances.
While FCEVs are less efficient than BEVs in terms of energy use, their zero tailpipe emissions and rapid refueling capability make them a compelling solution, especially for vehicles that require a quick turnaround and long-distance range, such as heavy-duty trucks and buses. As the technology evolves and the production of “green” hydrogen becomes more efficient and cost-effective, FCEVs are poised to play a vital role in the future of sustainable transportation.